Mars' atmosphere: Facts about composition and climate
Mars' atmosphere is over 100 times thinner than Earth's.

Mars' atmosphere is over 100 times thinner than Earth's and is primarily composed of carbon dioxide, nitrogen and argon gases.
Oxidized dust particles kicked up from the Martian surface fill the atmosphere turning Mars' skies a rusty tan color, according to NASA.
Water exists on Mars but the atmosphere is too thin for it to last long on the surface in a liquid state. Instead, water on Mars is found below the surface of the polar regions as water-ice and also as seasonal briny water flows down hillsides and crater walls.
Despite Mars' thin atmosphere, the Red Planet still exhibits a dynamic climate and extreme weather events including impressive dust storms and even snow! But Mars hasn't always been this way. NASA's MAVEN mission scientists reported that Mars once had a thick atmosphere that could have supported surface liquid water on the surface for extended periods of time.
Mars atmosphere composition
According to ESA, Mars' atmosphere is composed of 95.32% carbon dioxide, 2.7% nitrogen, 1.6% argon and 0.13% oxygen. The atmospheric pressure at the surface is 6.35 mbar which is over 100 times less Earth's. Humans therefore cannot breathe Martian air.
For crewed Mars exploration efforts, we need to find a way to generate oxygen from the thin, carbon dioxide atmosphere and an experiment carried out on NASA's Perseverance rover has demonstrated it is possible. On Apr. 20, 2021, the rover used its MOXIE (short for "Mars Oxygen In-Situ Resource Utilization Experiment") to successfully convert carbon dioxide to oxygen on Mars. "MOXIE has more work to do, but the results from this technology demonstration are full of promise as we move toward our goal of one day seeing humans on Mars." Jim Reuter, associate administrator of NASA's Space Technology Mission Directorate, said in a statement.
Martian climate and weather

Early in its history Mars had a thick enough atmosphere for water to run on its surface. According to NASA, some surface features suggest that Mars experienced huge floods about 3.5 billion years ago.
Orbital pictures show vast river plains and possible ocean boundaries, while several Mars rovers have found evidence of water-soaked rocks on the surface (such as hematite or clay). However, for reasons that are still poorly understood, the Martian atmosphere thinned.
Mars is much colder than Earth due to the thin atmosphere and the fact it is farther from the sun. The average temperature on Mars is about minus 80 degrees Fahrenheit (minus 60 degrees Celsius), although it can vary from minus 195 F (minus 125 C) near the poles during the winter to as much as a comfortable 70 F (20 C) at midday near the equator.
Like Earth, Mars has four seasons but due to the Red Planet's eccentric orbit, the length of each season varies more than on Earth, according to NASA science.
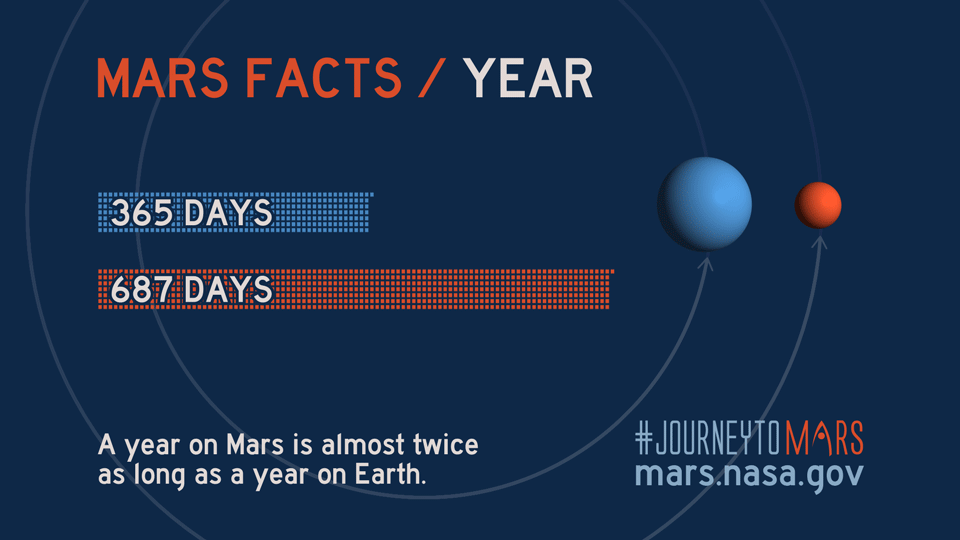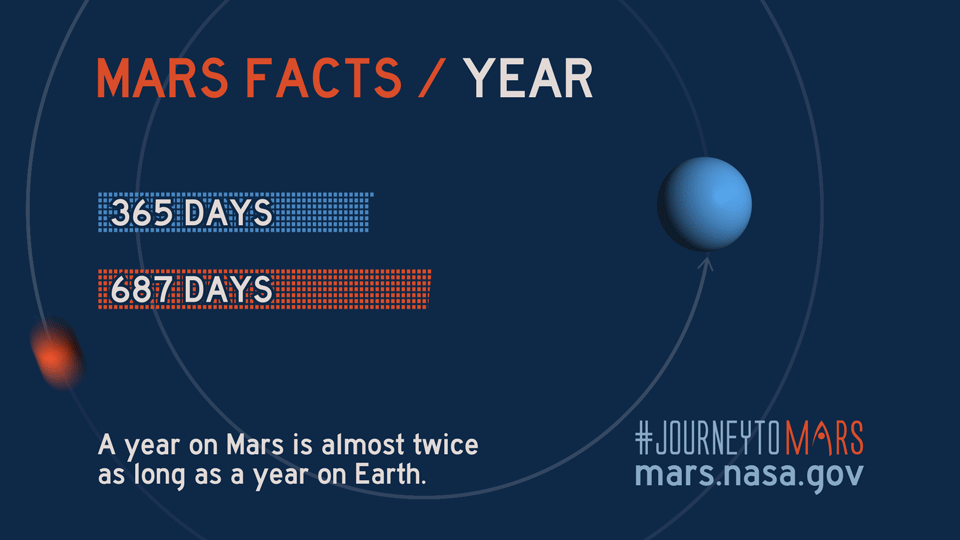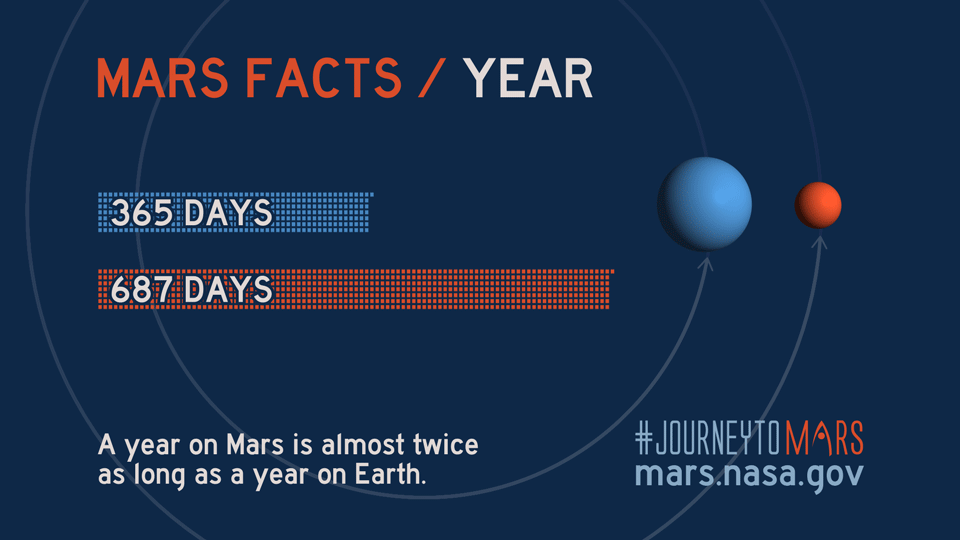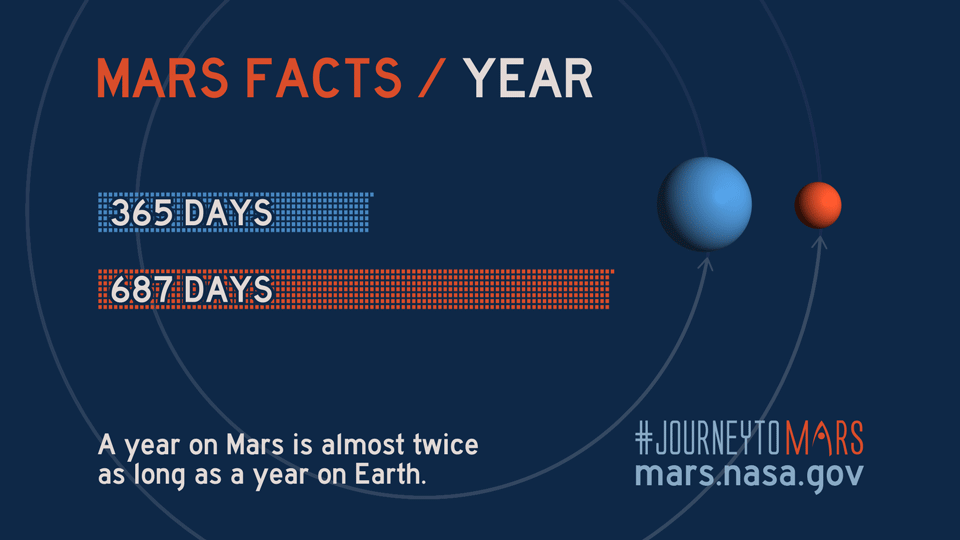
| Season (Northern Hemisphere) | Length of Martian season (sols) | Length of Earth season (days) |
|---|---|---|
| Spring | 194 | 93 |
| Summer | 178 | 93 |
| Autumn | 142 | 90 |
| Winter | 154 | 89 |
Mars' ice caps — made of water ice and carbon dioxide, shrink and grow in response to the seasons. These seasonal changes to the ice caps affect Mars' atmosphere, which responds as one large interconnected system, according to a statement from ESA. "The lower and middle levels of Mars' atmosphere appear to be coupled to the upper levels: there's a clear link between them throughout the martian year," says Beatriz Sánchez-Cano, a planetary scientist at the University of Leicester, UK.
"Each winter, up to a third of the mass in Mars' atmosphere condenses to form an icy layer at each of the planet's poles. Every spring, some of the mass within these caps sublimates to rejoin the atmosphere, and the caps visibly shrink as a result," ESA stated.
Giant dust devils routinely kick up the oxidized iron dust that covers Mars' surface. Dust is also a permanent part of the atmosphere, with higher amounts of it in the northern fall and winter, and lower amounts in the northern spring and summer. The dust storms of Mars are the largest in the solar system, capable of blanketing the entire planet and lasting for months. These usually take place in the spring or summer.
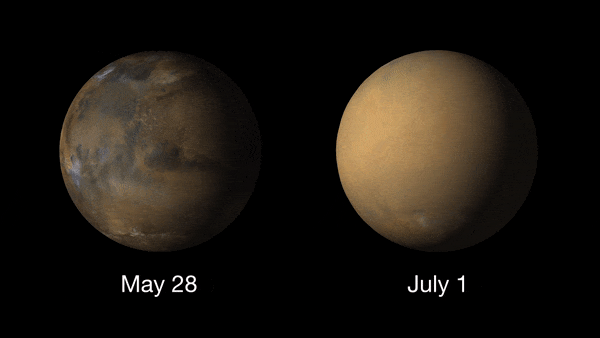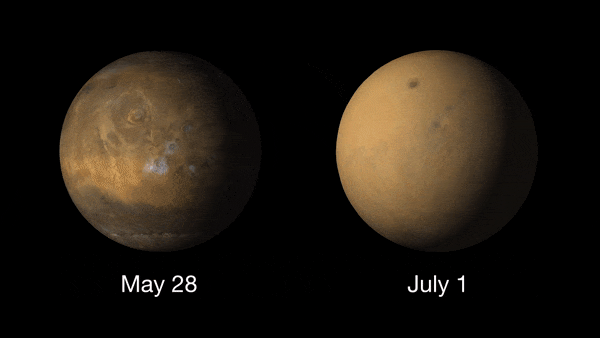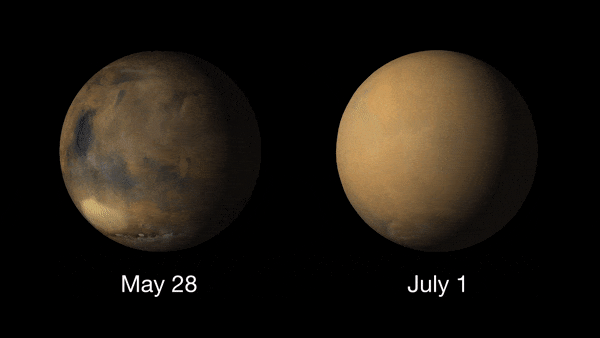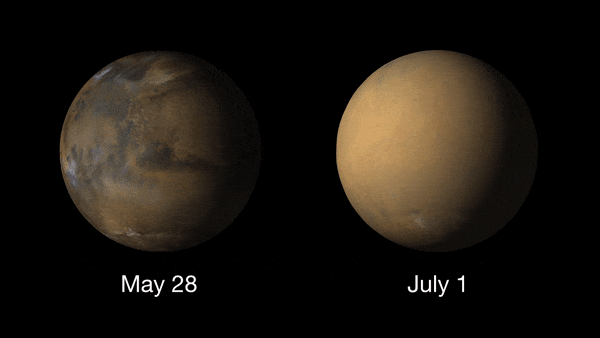
These dust storms can play havoc with Mars exploration missions and can even ground flights (yes Earth isn't the only planet where flights can be delayed due to poor weather!). NASA's Ingenuity Mars helicopter was due to make its 19th flight on the Red Planet on Jan. 5, 2022, when a dust storm near Jazero Crater had other plans.
"Most notable was a sharp drop in air density — about a 7% deviation below what was observed pre-dust storm," Jonathan Bapst and Michael Mischna, of Ingenuity's weather/environment team, said in a statement. "This observed decrease would have put density below the lower threshold of safe flight and would have imparted undue risk to the spacecraft. We also observed the effect of dust in the amount of sunlight absorbed by Ingenuity's solar array, which fell well below normal 'clear sky' levels, a drop of about 18%." Over a month passed until Ingenuity was clear to fly again, finally acing its 19th flight on Feb. 8, 2022.
One theory as to why dust storms can grow so big on Mars starts with airborne dust particles absorbing sunlight, warming the Martian atmosphere in their vicinity. Warm pockets of air flow toward colder regions, generating winds. Strong winds lift more dust off the ground, which in turn heats the atmosphere, raising more wind and kicking up more dust. A 2015 study further suggested that the momentum of Mars — which is affected by other planets — generates planet-circling dust storms when that momentum is at its greatest during the early part of the dust storm season.
At times, it even snows on Mars. The Martian snowflakes, made of carbon dioxide rather than water, are thought to be very small particles that create a fog effect rather than appearing as falling snow. The north and south polar regions of Mars are capped by ice, much of it made from carbon dioxide, not water.
How did Mars lose its atmosphere?

At some point in Mars' history, the Red Planet lost much of its atmosphere, transforming it from a warm wet world to the cold arid plains we see today, said ESA in a statement
Mars' atmosphere continues to "leak" out into space but how?
The leading theory is that Mars' light gravity, coupled with its lack of global magnetic field, left the atmosphere vulnerable to pressure from the solar wind, the constant stream of particles coming from the sun. Over millions of years, the sun's pressure stripped the lighter molecules from the atmosphere, thinning it out. This process is being investigated by NASA's MAVEN (Mars Atmosphere and Volatile Evolution) mission. Other researchers hypothesize that perhaps a giant impact by a small body would have stripped the atmosphere away.
Mars atmosphere expert Q&A
We spoke to Timothy A. Livengood, an observational planetary astronomer about Mars' atmosphere.

Timothy Livengood is an observational planetary astronomer who studies the atmospheres of planetary bodies. He is currently working on projects studying the atmosphere of Mars and Jupiter as well as volatile gases on the moon.
How and why might the composition of Mars's atmosphere change periodically (for instance, over the course of a day, seasonally, etc.)?
The atmosphere of Mars changes over the course of a day because the ground gets extremely cold at night on Mars, down to around minus 160°C. At such cold temperatures, both major and minor constituents of the atmosphere might either condense (snow, frost) or just stick to the soil grains a lot more than they do at warmer temperatures. Because of differing condensation temperatures and "stickiness", the composition can change significantly with the temperature.
During the day, the gases are released from the soil at varying rates as the ground warms, until the next night. It stands to reason that similar processes happen seasonally, as the water (H2O) and carbon dioxide (CO2) condense as frost and snow at the winter pole in large quantities while sublimating (evaporating directly from solid to gas) at the summer pole.
It gets complicated because it can take quite a while for gas released at one pole to reach the other. Many species may be more sticky to soil grains than to ice of the same material, so for those chemicals, the diurnal change could be more significant than the seasonal change.
The seasonal variation changes the global air pressure on Mars and changes which gases are in the air. On Mars, the air pressure can change globally by ±8%, depending on the season. The result is that there's no specific place, time of day, or season that fully represents THE composition of Mars' atmosphere. Finally, sunlight drives atmospheric chemistry by way of solar ultraviolet light breaking down CO2 and H2O molecules that are then free to react in new ways. As a result, the changing pressure and abundance of H2O and CO2 changes the abundance of carbon monoxide (CO), oxygen (O2), ozone (O3), and other trace species.
Why do scientists think that Mars used to have a much thicker atmosphere?
The most obvious reason to think that Mars once had a much more dense atmosphere is that there are clear signs all over Mars of erosion by water in processes that occur on Earth but could not occur on Mars as it is today.
There are river channels, eroded valleys, rocks worn into round river cobbles and dumped at the end of valleys, consistent with the way that rivers deposit rocks. More has changed than just the amount of water. Under current conditions, a puddle of liquid water at the surface would quickly evaporate, or freeze and then sublimate, because of the extremely low air pressure. For water to have once persisted long enough to flow in large quantities and to pool, the air pressure had to be much greater.
From the surface, rovers have detected salt minerals layered in patterns that are consistent with the way that salty water forms lakes on Earth that evaporate over long periods of time in places like the Great Salt Lake or the Dead Sea, and in fully dried lakes such as salt flats in the American West, in Africa, and numerous other places. From orbit, we have observed "bathtub rings" in large depressions in the surface and detected minerals that can only form when materials dissolve in water and have time to react with each other and accumulate in large quantities.
What we lack is a good estimate of how much more dense the atmosphere once was. An important clue is in the isotopes of atmospheric gas atoms. Atoms in gases exist in different versions, called isotopes, that each have the same number of protons and electrons, but different numbers of neutrons in the nucleus. There are two stable versions of carbon, and three stable versions of oxygen.
The ratio that we measure now between the abundance of stable isotopes depends on the original ratio when the planet formed, and then how it changed over time as heavy or light isotopes (more or fewer neutrons) were removed from the atmosphere by chemistry to form solids, or escaped to space. Hydrogen, nitrogen, and argon atoms are all enriched in heavy isotopes, consistent with losing a lot of gas to space, which removes the light isotopes faster than the heavy ones. Carbon and oxygen, which make the main atmospheric gas, carbon dioxide (CO2), are much more confusing.
Measurements from the Phoenix lander and Curiosity rover show the heavier isotopes are enriched, but they do not agree with each other, and neither agrees with Viking measurements from the 1970's that showed no enrichment. We need an estimate of what the isotope ratios actually are today, what they once were in the deep past, and how quickly each kind of isotope is lost from the atmosphere. With those things, we can estimate how much atmosphere has been lost and thus estimate how much atmosphere Mars once had. It will be a slow and messy process of gradually improving measurements, vigorous disagreements, improving understanding, and comparing results from entirely different methods to hone in on the truth.
We also have another published set of measurements, made by telescope from Earth, that suggests that the relative amount of heavy isotopes in CO2 changes with the ground temperature over the course of the day. These measurements were interpreted by me as due to the heavy versions of CO2 sticking to soil grains at low temperatures more effectively than the light isotopes, so that the isotope ratios change throughout the day and only get close to the real value of heavy isotope enrichment when the ground is warmest. That would suggest that the reason none of the previous measurements agree with each other is because they were all acquired at different times of day, under different climate conditions, at different seasons. However, my interpretation could be wrong.
The telescopic measurements need to be tested with other measurements, from Earth and at the surface of Mars, to determine whether it is a real process, whether it is routine, or whether the interpretation is a misunderstanding of the data. A powerful argument against my interpretation is that laboratory measurements do not yet show any process that could be effective enough to make this happen to the extent that the measurements appear to show. Also, we don't have the data (yet) to show whether there is a significant seasonal effect in atmospheric isotope ratios on Mars. There probably is not, because lab measurements show that freezing pure ices of CO2 does not result in significantly changing isotope ratios in the remaining gas. But that could be incorrect in the complex environment of a planet's surface.
Besides thickness, how might Mars's atmosphere be different from its atmosphere when it was a more Earth-like planet?
Mars once was a more Earth-like planet, but it was the ancient Earth that it resembled, a planet with a CO2-rich atmosphere and no free oxygen, with extensive oceans that may have been frozen-over much of the time. We don't know for certain what chemicals were in the atmosphere of Earth besides CO2, N2, and H2O prior to the rise of widespread oxygen-producing photosynthesis that radically altered Earth's atmosphere. There could have been gases like methane (CH4) and ammonia (NH3), sulfur-bearing gases, and other gases that are not common today. We don't see those gases in Mars' atmosphere today, either, but possibly they were there in the past. The Sun has also been slowly increasing in luminosity, so that Mars and Earth of a few billion years ago had less sunlight to warm them, but more CO2 in the atmosphere to hold in the heat. Mars of a few billion years ago probably had exposed lakes and oceans, and probably had rain.
Given how much uncertainty there is about how Earth's atmosphere may have been different when it was a younger and "less Earth-like" planet, it is awfully hard to make an educated guess as to what Mars' ancient atmosphere may have been like. Earth is particularly challenging because there has been constant chemistry and weathering as well as plate tectonics remaking the planet's surface over billions of years, minerals reacting with the atmosphere and sinking to the bottom of the oceans, then buried by tectonics. As we continue to gather new information about Mars, it may end up being our best guide to what Earth once was like, because the water chemistry on Mars, and plate tectonics, turned off a long time ago, leaving the rocks of that time intact for us to examine.
How can scientists learn more about what the Martian atmosphere used to be like?
The rocks remember. Many types of rock can only form at the surface, where water dissolves both the minerals and the atmosphere so that they can react with each other to form new compounds that can condense to form new minerals. Examples on Earth include limestones (carbonate minerals) and many iron-bearing minerals.
The goal of the Perseverance rover mission on Mars now is to obtain samples of surface rocks in the mouth of an ancient river by drilling into the interior of rocks in the streambed to extract pencils of material. The exterior of these rocks has been weathered by the modern Mars environment, but the interior represents the chemical conditions under which the rock formed. That includes the relative rate at which different chemical reactions happened (a thermometer), what was dissolved in the water (including the atmosphere), and what isotope ratios were present for different elements.
Perseverance leaves "caches" of these samples in sealed containers. Sometime in the next decade, a contingent of spacecraft will be sent to retrieve the samples from the surface and launch them to Mars orbit, then take them from Mars orbit back to Earth. It is a tremendously ambitious and complex undertaking, involving many first-time events. Much of what we need to investigate to understand ancient Mars is far beyond the capability of instruments that can be made small, light, and efficient enough to fit onto a spacecraft. We need these samples from Mars to be returned to Earth where we can use the great variety of instruments we have here to study them completely.
Additional resources
If you would like to see where NASA's Maven mission is right now check out their mission page with a real-time simulated view of the spacecraft. Interested in terraforming? Check out our article on whether we can terraform Mars to make it more hospitable. Explore the science of becoming "Interplanetary" with this engaging article from Interesting Engineering.
Bibliography
- Ramstad, Robin, et al. "Global mars‐solar wind coupling and ion escape." Journal of Geophysical Research: Space Physics 122.8 (2017): 8051-8062.
- Sánchez‐Cano, Beatriz, et al. "Spatial, seasonal, and solar cycle variations of the Martian Total Electron Content (TEC): Is the TEC a good tracer for atmospheric cycles?." Journal of Geophysical Research: Planets 123.7 (2018): 1746-1759.
- Smith, David E., Maria T. Zuber, and Gregory A. Neumann. "Seasonal variations of snow depth on Mars." Science 294.5549 (2001): 2141-2146.
- Banfield, Don, et al. "The atmosphere of Mars as observed by InSight." Nature Geoscience 13.3 (2020): 190-198.
Join our Space Forums to keep talking space on the latest missions, night sky and more! And if you have a news tip, correction or comment, let us know at: community@space.com.
Get the Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Daisy Dobrijevic joined Space.com in February 2022 having previously worked for our sister publication All About Space magazine as a staff writer. Before joining us, Daisy completed an editorial internship with the BBC Sky at Night Magazine and worked at the National Space Centre in Leicester, U.K., where she enjoyed communicating space science to the public. In 2021, Daisy completed a PhD in plant physiology and also holds a Master's in Environmental Science, she is currently based in Nottingham, U.K. Daisy is passionate about all things space, with a penchant for solar activity and space weather. She has a strong interest in astrotourism and loves nothing more than a good northern lights chase!
- Elizabeth HowellStaff Writer, Spaceflight
- Tim SharpReference editor
